Solids are the materials having definite volume and shape. Solids can be described in two different categories. These are discussed below:
1. Crystalline solids
2. Amorphous Solids
In solids, the atoms and molecules are bound with strong forces. This strong force attraction results in the appropriate shape of solids.
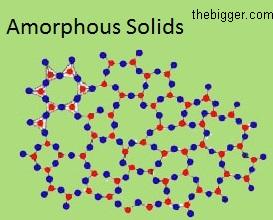
Crystalline solids: Solids having a particular external geometrical shape are known as crystalline solids. The arrangement of atoms, molecules and ions is in a three dimensional structure. Some common examples of crystalline solids are Calcite, Rock salt, Mica, Diamond etc.
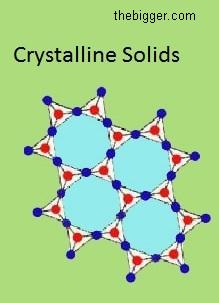
Amorphous Solids: Those solids which have no particular external shape are known as amorphous solids. No particular arrangement of atoms, molecules and ions is there in amorphous solids. Examples of amorphous solids are Plastic, Rubber, etc.