We know that work differential dw is calculated as:
dw= PdV ……………. (1)
since volume V is a state function i.e. it is independent of the path [V=f (T,P)] hence dV is an exact differential.Therefore
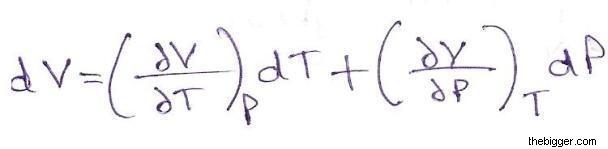 ……. (2)
……. (2)
For an ideal gas, we know that PV = RT which means:
V= RT/P
Hence,
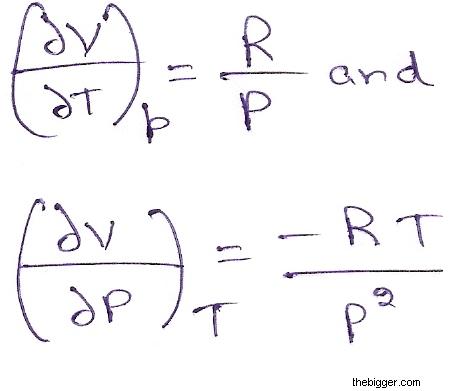 ……… (3)
……… (3)
Substituting the Equations (1) and (2) in equation (1), we get:
dw = PdV = RdT – (RT/P) dP = RdT – VdP …………………. (4)
Applying the Euler reciprocal relation to equation (4), we get
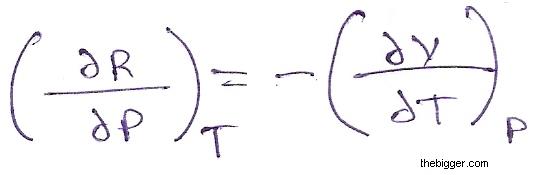
Since R is the gas constant, hence (∂R/∂P) T = 0 ……… (5)
Accordingly, from equation (5), (∂V/∂T) P = 0
But it brings contradiction as there is always a change in volume with temperature at constant pressure. This leads to the conclusion that dw is not an exact differential.
