According to the principle of photochemical activation, only that part of the light which is absorbed by any system can cause photochemical change or reaction. However this is not essential that every time, the energy which is absorbed by any system can bring about a photochemical reaction.
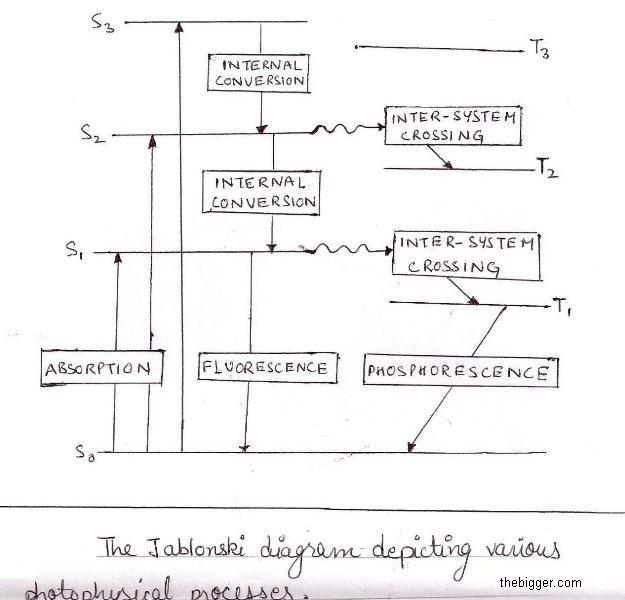
Also the light absorbed by any system may be re-emitted almost at the same time, either in one step or in more steps. This phenomenon is called fluorescenes. Also sometimes the light absorbed by any system may re-emit the energy slowly or even after the removal of source light, this phenomenon is called phosphorescenes. Process of Fluorescenes, phosphorescenes is best explained with the help of Jablonski diagram.
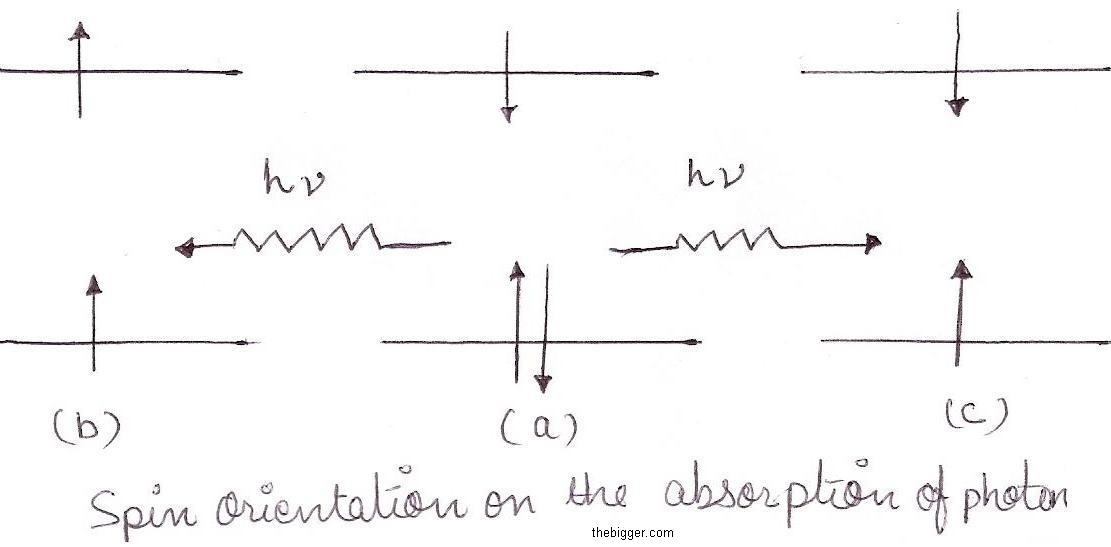
In order to understand the diagram, we have considered some terminology. As most molecules contain even number of electrons hence they are in ground state, also all the electrons are paired with other electrons. The equation 2S+1, where S is known as the spin multiplicity means total number of electron spin. When the spins are paired in opposite direction as shown in fig. 1(a), the upward orientation of electron movement canceled the downward orientation , which makes S=0, this is illustrated below :
s1 = 1/2; s2 = -1/2 so that
S = s1 + s2 = 1/2-1/2 =0
Hence, 2S+1 =1. We express it by saying that the molecule is in the singlet ground state. When upon absorption of energy hv by a photon, one of the paired electron get excited and goes to higher level, the spine orientation of two single electrons may be either parallel or anti parallel, as shown in fig. 1(b) and fig. 1(c). If the spin orientation are parallel as in fig. 1(b), then
S = s1 + s2 =1/2 + 1/2 =1 so that
2S + 1 = 3
Thus, we express it by saying that the molecule is in the triplet excited state with spin multiplicity 3.If however, the spins are anti parallel as shown in fig. 1(c), then
S = s1 + s2 =1/2 – 1/2 =0 so that
2S + 1 =1
Thus, we express it by saying that the molecule is in the singlet excited state with spin multiplicity 1.
So far we have discovered that electron can jump to any of the higher state depending upon the amount of energy of they get absorbed, by this we get a series of singlet excited states, Sn where n =1,2,3,4 … and a series of triplet excited states, Tn where n =1,2,3,4 … .Thus S1, S2, S3 etc. similarly, T1, T2, T3, etc, are called first triplet excited state, second triplet excited state, third triplet excited state and so on.
Also note that according to quantum mechanism a singlet excited state has higher energy than the triplet excited state. Hence the energy sequence is like this:
ES1 > ET1;
ES2 > ET2;
ES3 > ET3; and so on.
We know that electron can jump to any of the higher state depending upon the amount of energy of they get absorbed, as shown in Jablonski diagram. Every singlet excited state, there are corresponding triplet excited state. Thus
A0 + hv ———> A*
Where A0 is the molecule in ground state and A* is the molecule in the excited state.
When the activated molecule returns to the ground state by eliminating energy at each level following types of processes takes place:
1. Non-radiative Transition: When an activated molecule returns from higher excited state to first state i.e. from S1 to T1. In this process there exists no emission of radiations that’s why it’s called Non-radiative Transition. The energy in the molecule lost in the form of heat to the environment.
2. Radiative Transition: When any activated molecule returns from siglet excited and triplet excited state to the ground state, in such transition there exists emission of radiations that’s why it’s called Radiative Transition.
3. Chemical Reactions: When any activated molecule loose energy chemically. Since the molecule present in the singlet excited state returns quickly to the ground state, then there is no chance to react chemically, but the molecule returns from triplet excited state to ground state has enough time to react chemically. This time interval provides the molecule to react chemically.
