Packing fraction is defined as a way of expressing the variation of isotopic mass from whole mass number (atomic mass).
This fraction can have positive or can have negative sign. A positive packing fraction describes a tendency towards instability. A negative packing fraction means isotopic mass is less than actual mass number. This difference is due to the transformation of mass into energy in the formation of nucleus. A plot of packing fraction against corresponding mass no’s of various elements is as shown.
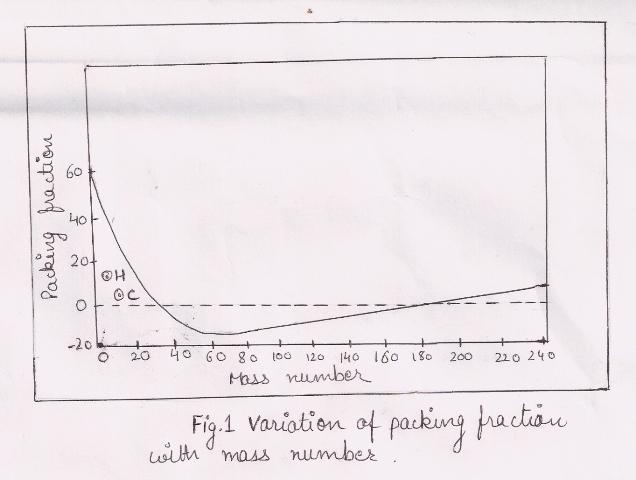
From figure it is clear that the elements which have less atomic mass (hydrogen, helium, carbon) do not fall on this curve. Therefore, these are stable atoms. Oxygen is highly stable. The packing fraction beyond mass number 200 becomes positive and increases with increase in mass number. It increases instability of these elements.


