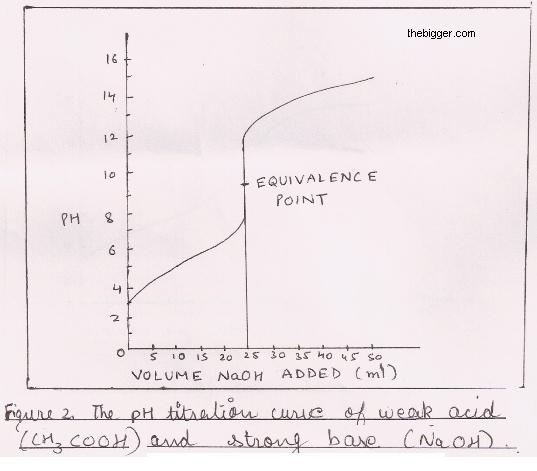
Titration of Weak Acid and a Strong Base:
For explaining titration of weak acid and a strong base let us consider an example of acetic acid i.e. CH3COOH which is a weak acid and sodium hydroxide i.e. NaOH which is a strong base. The reaction can be shown as:
CH3COOH (aq) + NaOH (aq) ———> CH3COONa (aq) + H2O (l)
The titration curve is shown in figure below. In this reaction, from the weak acid we have the free H+ ion which is neutralized by OH– ions from the base. Due to this neutralization the pH value increases marginally. When we are observing the equivalence point, we see a large increase in pH but the curve slope is not that steep as we found it in the case of strong base and strong acid titration. At equivalence point the pH is not 7 because the sodium acetate formed after the neutralization reacts with water to give OH– ions. This decreases H+ ions concentration and pH is about 8.7 at equivalence point. Near the equivalence point pH change is observed from about 8.0 to 10.0 by the addition of small amount of NaOH. Due to this is becomes mandatory to use an indicator having pH in alkaline range. Phenolphthalein is therefore the one which is most commonly used.