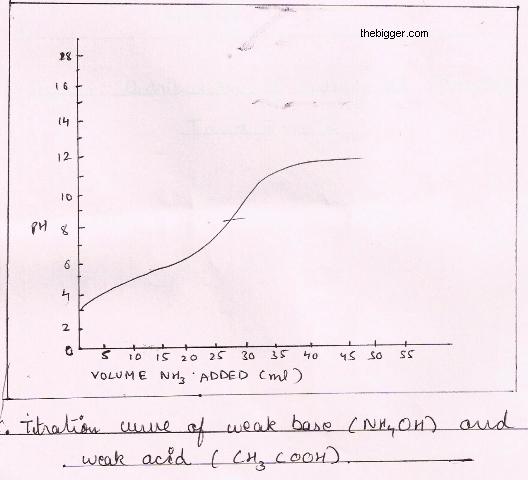
Titration of Weak Acid and Weak Base
For explaining this type of titration let us consider an example of acetic acid i.e. CH3COOH which is a weak acid and ammonium hydroxide i.e. NH4OH which is a weak base. The reaction can be shown as:
CH3COOH (aq) + NH4OH (aq) —-> CH3COO NH4(aq)+ H2O(l)
The pH curve is shown in figure below. In this case we don’t have any sharp equivalence point with common indicators but with a mixed indicator a sharp color change over limited pH range may sometimes be used. Such types of titrations are not commonly carried out in the laboratory.