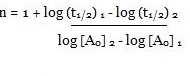Consider the two different initial concentrations and finding their half lives, the order of reaction (n) can be calculated as follows:
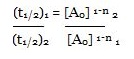
Taking log on both sides we will get:
log (t1/2) 1 – log (t1/2) 2
= n – 1 (log [A0] 2 – log [A0] 1)
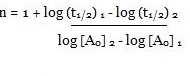
Consider the two different initial concentrations and finding their half lives, the order of reaction (n) can be calculated as follows:
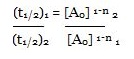
Taking log on both sides we will get:
log (t1/2) 1 – log (t1/2) 2
= n – 1 (log [A0] 2 – log [A0] 1)