Pi (π) Bonds
When sideways overlapping or lateral overlapping of the two atoms take place is known as lateral overlap of their ‘p’ orbital, and the bond formed is known as covalent pi (π) bond. The electron density is concentrated in the region perpendicular to the bond axis in a pi bond.
Due to partial overlapping of orbital, the bond formed which is pi is weak. If the ‘p’ orbitals are parallel then there will be complete sideways overlapping and hence the pi bond will be strong. This can be possible only when the atoms involved of the molecule are in the same plane. This means there is no rotation of the atom with respect to other which is formed on pi bond.
π is a Greek letter. It is known as π because it is formed by p-orbital.
The formation of Pi Bond is illustrated with the help of following example:
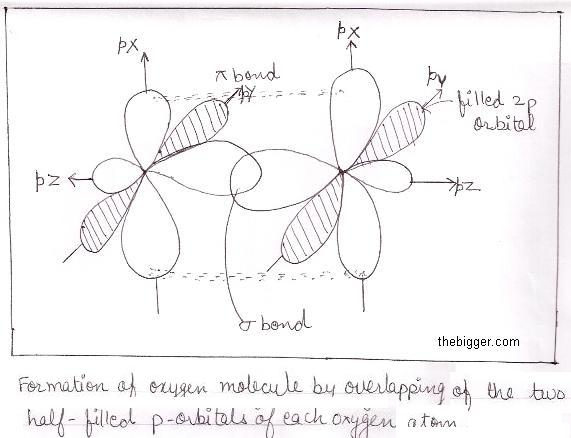
Formation of oxygen (O2) Molecule:
The electronic configuration of oxygen having Z=8 is 1s2 2s2 2p2x 2p1y 2p1z.
Each atom of oxygen has 6 (six) electrons in its valence shell. To achieve the nearest noble gas configuration, it needs 2 more electrons. Therefore sharing of two pairs of electrons by the two oxygen atoms occurs to form one molecule of oxygen.
Hence oxygen molecule is formed by double bond comprising of one sigma and one Pi-bond as shown below
Formation of Nitrogen (N 2) Molecule:
Nitrogen atom has 5 (five) electrons in its valence shell. To acquire a stable configuration of the nearest noble gas (neon), it requires three more electrons . This is done by sharing three pairs of electrons as shown below
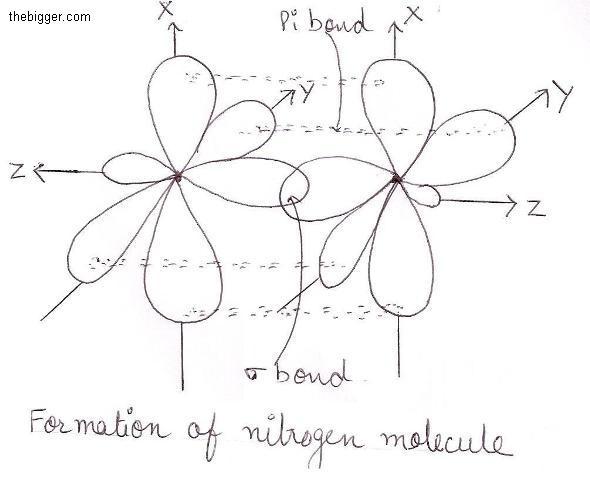
Hence in nitrogen molecule is formed by triple bond made up of one sigma bond and two Pi bonds.
