Resonance Energy
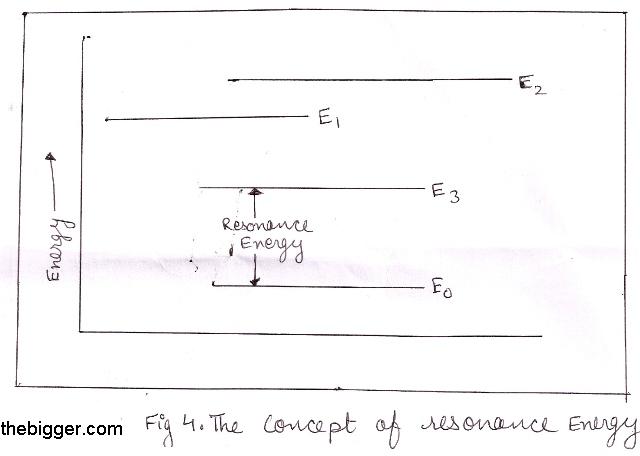
The difference between the actual energy of the molecule and that of most stable resonating structure is known as Resonance Energy.
Let us suppose that a molecule have three resonating structures which have energy E1, E2, E3 associated with them. From these three energies, E3 is having lowest energy which corresponds to the most stable resonating structure. If the actual energy of the molecule is E0 which is determined experimentally then:
Resonance Energy = E 3 – E 0
Rules to write the resonance structure:
1. Position of nuclei must be the same in all structures otherwise they will form isomers.
2. The Total number of electrons and total charge must be constant.
3. When separating charge (giving rise to ions), usually structures have little contribution where negative charges are on less electronegative elements, but this may not be true if additional bonds are gained.
4. Resonance hybrids cannot be made to have lower energy than the actual molecules.
5. Resonance hybrids must have the same number of unpaired electrons (if exists).
