The relative energies of molecular orbitals depend upon the following two factors:
(i) Atomic orbitals energies for the combination to form molecular orbitals.
(ii) The extent of overlapping between the atomic orbitals.
The greater the overlap, the more the bonding orbital is lowered and the anti-bonding orbital is raised in energy relative to atomic orbitals. For e.g., the extent of overlapping in case of sigma – orbital is more than that in pi – orbital. Consequently, the energy of a σ2pz is lower than the energy of bonding π2px or π2py MOs.
Now, 1s atomic orbitals of two atoms form two molecular orbitals designated as σ1s and σ*1s. The 2s and 2p orbitals (eight atomic orbitals on two atoms) form four bonding molecular orbitals and four anti-bonding molecular orbitals as:
Bonding MOs:
σ2s, σ2p z, π2p x, π2p y
Anti-bonding MOs:
σ *2s, σ *2p z, π*2p x ,
π*2p y
The energy levels of these molecular orbitals have been determined experimentally by various methods. The order of increasing energy of molecular orbitals obtained by the combination of 1s, 2s and 2p orbitals of two atoms is:
s1s, s*1s, s2s, s*2s, s2pz , p2Px = p2py , p*2px = p*2py , s*2pz
Energy increases ———————————->
But for molecules O2 onwards (O2, F2), the first order of energies of MOs is correct.
Thus, for diatomic molecules of second period (Li2 to Ne2), there are two types of energy levels of Mos. For molecules Li2, Be2, B2, C2 and N2 the molecular orbital energy level diagram
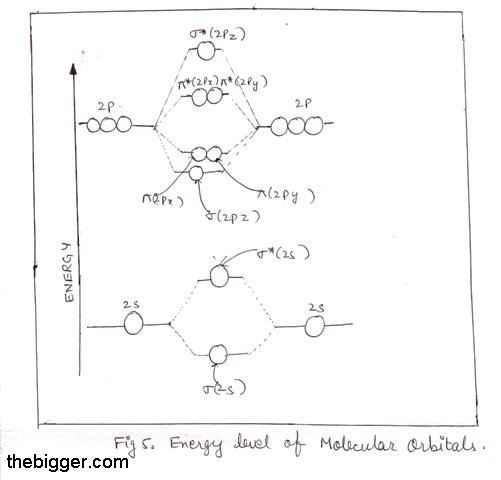
In the diagram, the molecular orbitals are place at the center and the atomic orbitals at the same level. The bonding molecular orbitals are at lower place and the antibonding molecular orbitals are higher as compared to atomic orbitals. This means bondind molecular orbitals are more stable than antibonding molecular orbitals.
